on/go™ COVID-19 Rapid Antigen At-Home Self Test approved for Over-The-Counter sales. Tech Enabled via Smart Phone Application of IOS and Android.
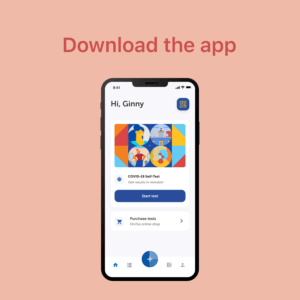

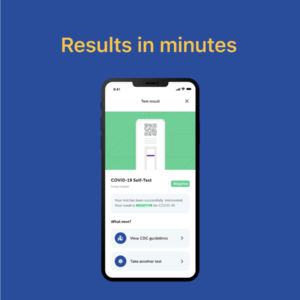
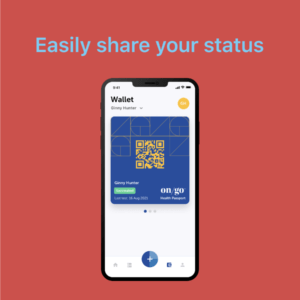
On/Go is a reliable, portable, and affordable over-the-counter COVID testing and tracing solution capable of detecting all known COVID-19 variants
Easy-to-use iOS and Android mobile apps guide users through an intuitive testing experience
For population managers, the new AI-powered platform seamlessly integrates with On/Go rapid COVID tests, offering a real-time solution to avoid costly shutdowns through rapid, targeted interventions that enable peace of mind
Manufactured in the U.S., On/Go has the ability to ship tens of millions of tests monthly and hundreds of millions of tests yearly, and is continuing to ramp up production rapidly
| Country of Origin | United States |
| Application | Rapid Test Kit – Self Test |
| Contents | (2) Test Devices, (2) Extraction Reagent Capsules, (2) Sterile Swabs, Package Insert, (1) Quick Reference Instruction For Use |
| FDA EUA No. | EUA210314/S001 |
| Storage | Room Temperature |
| Test Format | Test Device |
| Test Method | Lateral Flow Immunochromatographic Assay |
| SKU | QRI-RCPM79-E |
| Test Type | Rapid Antigen Detection |
| Time to Results | 10 Minute Results |
| Kit Packing | 2 Tests |
| Case Packing | 232 Kits (464 Tests) |
| Application | At-Home OTC COVID-19 Test |
| Pallet Packing | 20 Cases (9,280 Tests / 4,640 Kits) |
- For Use Under an Emergency Use Authorization (EUA) Only: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-antigen-diagnostic-tests-sars-cov-2
- The on/go™ At-Home OTC COVID-19 Test is intended for the qualitative detection of nucleocapsid proteins from SARS-CoV-2 from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over two or three days with at least 24 hours and no more than 36 hours between tests
- This test is authorized for nonprescription home use with self-collected (unobserved) direct anterior nasal(NS) swab specimens from individuals aged 14 years and older or with adult-collected anterior NS samples from individuals aged 2 years or older
- Individuals should provide all results obtained with this product to their healthcare provider for public health reporting
- This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens
- The test is intended to be read at 10 minutes; If the test is read before this or is read more than 5 minutes after the indicated read time, results may be inaccurate and the test should be repeated